RECENT CASE STUDY
PHARMA COMPANY CONSOLIDATES ALL DATA SOURCES & RELATIONSHIPS FOR MEDICAL TERMINOLOGY INTO A SINGLE ENTERPRISE DATA CATALOG
THE COMPLICATION
𝖠 𝗅𝖺𝗋𝗀𝖾 𝗉𝗁𝖺𝗋𝗆𝖺 𝖼𝗈𝗆𝗉𝖺𝗇𝗒 𝗁𝖺𝖽 𝖺 𝗅𝗈𝗇𝗀 𝖺𝗇𝖽 𝗆𝖺𝗇𝗎𝖺𝗅 𝗉𝗋𝗈𝖼𝖾𝗌𝗌 𝗍𝗈 𝗌𝗍𝖺𝖻𝗅𝗂𝗌𝗁 𝗂𝗆𝗉𝖺𝖼𝗍 𝖺𝗇𝖺𝗅𝗒𝗌𝗂𝗌 𝖻𝗈𝗍𝗁 𝗎𝗉𝗌𝗍𝗋𝖾𝖺𝗆 𝖺𝗇𝖽 𝖽𝗈𝗐𝗇𝗌𝗍𝗋𝖾𝖺𝗆 𝗍𝗈 𝖽𝖾𝗍𝖾𝗋𝗆𝗂𝗇𝖾 𝗇𝖾𝖼𝖾𝗌𝗌𝖺𝗋𝗒 𝖼𝗁𝖺𝗇𝗀𝖾𝗌 𝗐𝗂𝗍𝗁 𝖾𝗏𝖾𝗋𝗒 𝗇𝖾𝗐 𝗋𝖾𝗅𝖾𝖺𝗌𝖾 𝗈𝖿 𝖼𝗅𝗂𝗇𝗂𝖼𝖺𝗅 𝗍𝖾𝗋𝗆𝗂𝗇𝗈𝗅𝗈𝗀𝗒.
𝖳𝗁𝖾 𝗋𝖾𝗅𝖾𝖺𝗌𝖾 𝗁𝖺𝗉𝗉𝖾𝗇𝗌 𝗍𝗐𝗂𝖼𝖾 𝖺 𝗒𝖾𝖺𝗋 𝖺𝗇𝖽 𝗍𝗁𝖾 𝗉𝗋𝗈𝖼𝖾𝗌𝗌 𝗂𝗌 𝗋𝖾𝗀𝗎𝗅𝖺𝗍𝖾𝖽.
𝖠 𝗁𝗂𝗀𝗁𝗅𝗒 𝗆𝖺𝗇𝗎𝖺𝗅 𝗉𝗋𝗈𝖼𝖾𝗌𝗌 𝗂𝗇𝗏𝗈𝗅𝗏𝗂𝗇𝗀 𝗍𝗁𝖾 𝗎𝗌𝖾 𝗈𝖿 𝗆𝗎𝗅𝗍𝗂𝗉𝗅𝖾 𝗌𝗉𝗋𝖾𝖺𝖽𝗌𝗁𝖾𝖾𝗍𝗌 𝗍𝗈 𝗉𝗋𝗈𝗏𝗂𝖽𝖾 𝖺 𝗌𝗎𝗆𝗆𝖺𝗋𝗒 𝗈𝖿 𝖼𝗁𝖺𝗇𝗀𝖾𝗌. 𝖳𝗁𝖾 𝗋𝖾𝗅𝖺𝗍𝗂𝗈𝗇𝗌𝗁𝗂𝗉𝗌 𝖻𝖾𝗍𝗐𝖾𝖾𝗇 𝗍𝗁𝖾 𝖽𝖾𝗍𝖾𝗋𝗆𝗂𝗇𝖾𝖽 𝗊𝗎𝖾𝗋𝗂𝖾𝗌 𝖺𝗇𝖽 𝗍𝗁𝖾 𝖺𝗇𝖺𝗅𝗒𝗌𝗂𝗌 𝖽𝗈𝗇𝖾 𝗐𝖺𝗌 𝗇𝗈𝗍 𝗉𝗈𝗌𝗌𝗂𝖻𝗅𝖾 𝗍𝗈 𝗏𝗂𝗌𝗎𝖺𝗅𝗂𝗓𝖾.
𝖰𝗎𝖾𝗌𝗍𝗂𝗈𝗇𝗌 𝗅𝗂𝗄𝖾 𝗐𝗁𝗒 𝗐𝖺𝗌 𝗍𝗁𝖾 𝖼𝗁𝖺𝗇𝗀𝖾 𝗇𝖾𝖾𝖽𝖾𝖽 , 𝗐𝗁𝗈 𝖺𝗉𝗉𝗋𝗈𝗏𝖾𝖽 𝗍𝗁𝖾 𝖼𝗁𝖺𝗇𝗀𝖾 𝖺𝗇𝖽 𝗐𝗁𝖾𝗇 𝗐𝖾𝗋𝖾 𝗇𝗈𝗍 𝗉𝗈𝗌𝗌𝗂𝖻𝗅𝖾 𝗍𝗈 𝖻𝖾 𝖺𝗇𝗌𝗐𝖾𝗋𝖾𝖽 𝗊𝗎𝗂𝖼𝗄𝗅𝗒 𝗈𝗋 𝖾𝖺𝗌il𝗒.
𝖣𝗂𝖿𝖿𝖾𝗋𝖾𝗇𝗍 𝖫𝖮𝖡 𝗇𝖾𝖾𝖽𝖾𝖽 𝗍𝗁𝖾 𝖽𝖺𝗍𝖺 𝗂𝗇 𝖽𝗂𝖿𝖿𝖾𝗋𝖾𝗇𝗍 𝖿𝗈𝗋𝗆𝖺𝗍𝗌 𝖿𝗈𝗋 𝖼𝗈𝗇𝗌𝗎𝗆𝗉𝗍𝗂𝗈𝗇 𝖺𝗇𝖽 𝖺𝗇𝖺𝗅𝗒𝗌𝗂𝗌.
THE SOLUTION
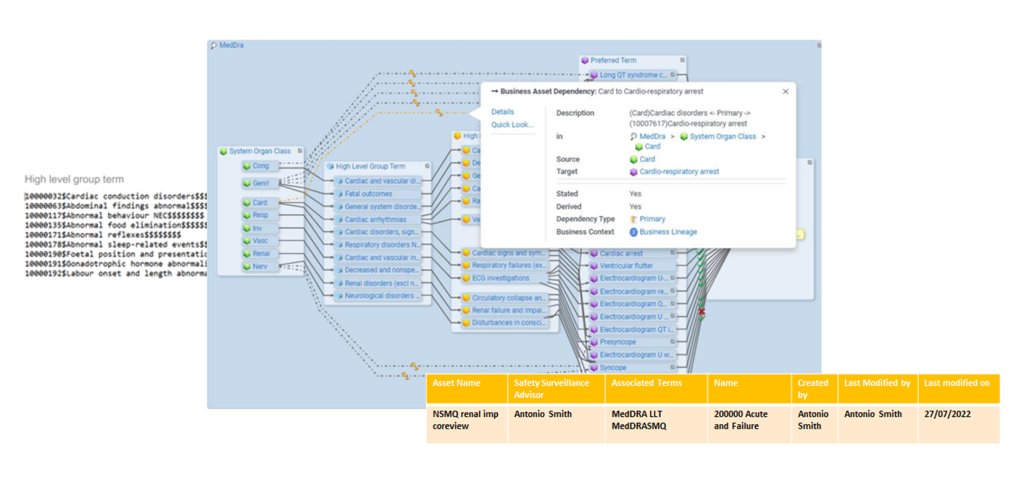
𝖳𝗁𝖾 𝗆𝖾𝖽𝗂𝖼𝖺𝗅 𝖽𝗂𝖼𝗍𝗂𝗈𝗇𝖺𝗋𝗒 𝗐𝖺𝗌 𝖼𝖺𝗍𝖺𝗅𝗈𝗀𝗎𝖾𝖽. 𝖠𝗅𝗅 𝖽𝖺𝗍𝖺 𝗌𝗈𝗎𝗋𝖼𝖾𝗌 𝗂𝗇𝗏𝗈𝗅𝗏𝖾𝖽 𝗐𝖾𝗋𝖾 𝖼𝖺𝗍𝖺𝗅𝗈𝗀𝗎𝖾𝖽 𝖺𝗇𝖽 𝗍𝗁𝖾 𝗋𝖾𝗅𝖺𝗍𝗂𝗈𝗇𝗌𝗁𝗂𝗉𝗌 e𝗌𝗍𝖺𝖻𝗅𝗂𝗌𝗁𝖾𝖽 𝖿𝗋𝗈𝗆 𝖺 𝗅𝗂𝗇𝖾𝖺𝗀𝖾 𝗉𝖾𝗋𝗌𝗉𝖾𝖼𝗍𝗂𝗏𝖾.
𝖣𝖺𝗍𝖺 𝗌𝖾𝖺𝗋𝖼𝗁 𝗂𝗇 𝖺 𝗌𝗂𝗇𝗀𝗅𝖾 𝗍𝗈𝗈𝗅 𝗐𝖺𝗌 𝖾𝗇𝖺𝖻𝗅𝖾d.
𝖳𝗁𝖾 𝗂𝗆𝗉𝖺𝖼𝗍 𝖺𝗇𝖺𝗅𝗒𝗌𝗂𝗌 𝗂𝗌 𝗇𝗈𝗐 𝖽𝗈𝗇𝖾 𝗂𝗇 𝖺 𝗌𝗂𝗇𝗀𝗅𝖾 𝗍𝗈𝗈𝗅 𝖺𝗇𝖽 𝗍𝗁𝖾 𝗉𝗋𝗈𝗉𝗈𝗌𝗂𝗍𝗂𝗈𝗇 𝗈𝖿 𝖼𝗁𝖺𝗇𝗀𝖾𝗌 𝗂𝗌 𝗀𝗈𝗏𝖾𝗋𝗇𝖾𝖽 𝖻𝗒 𝖽𝖺𝗍𝖺 𝗈𝗐𝗇𝖾𝗋𝗌𝗁𝗂𝗉 𝖺𝗇𝖽 𝖺𝗉𝗉𝗋𝗈𝗏𝖺𝗅 𝖿𝗅𝗈𝗐𝗌. 𝖳𝗁𝖾𝗌𝖾 𝗂𝗌 𝖺𝗇 𝖺𝗎𝖽𝗂𝗍 𝗍𝗋𝖺𝗂𝗅 𝗍𝗈 𝗌𝖾𝖾 𝖼𝗁𝖺𝗇𝗀𝖾𝗌 𝖺𝗇𝖽 𝗏𝖾𝗋𝗌𝗂𝗈𝗇𝗌.
𝖳𝗁𝖾 𝖺𝖻𝗂𝗅𝗂𝗍𝗒 𝗍𝗈 𝗌𝖾𝖺𝗋𝖼𝗁 𝖿𝗈𝗋 𝖺 𝗍𝖾𝗋𝗆 𝖺𝗇𝖽 𝗌𝖾𝖾 𝗁𝗈𝗐 𝗆𝖺𝗇𝗒 𝗊𝗎𝖾𝗋𝗂𝖾𝗌 𝖺𝗋𝖾 𝖺𝖿𝖿𝖾𝖼𝗍𝖾𝖽 𝗐𝖺𝗌 𝖼𝗈𝗇𝖿𝗂𝗀𝗎𝗋ed 𝗍𝗈 𝖿𝖺𝖼𝗂𝗅𝗂𝗍𝖺𝗍𝖾 𝗍𝗁𝖾 𝗂𝗆𝗉𝖺𝖼𝗍 𝖺𝗇𝖺𝗅𝗒𝗌𝗂𝗌 𝗉𝗋𝗈𝖼𝖾𝗌s. 𝖳𝗁𝖾 𝗉𝗋𝗈𝖼𝖾𝗌𝗌 𝖻𝖾𝖼𝖺𝗆𝖾 𝗌𝗂𝗆𝗉𝗅𝖾𝗋, 𝗌𝗁𝗈𝗋𝗍𝖾𝗋 𝖺𝗇𝖽 improved 𝗀𝗈𝗏𝖾𝗋𝗇𝖺𝗇𝖼𝖾 process.